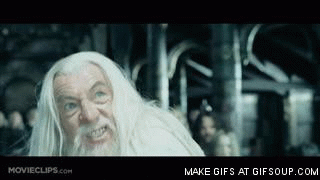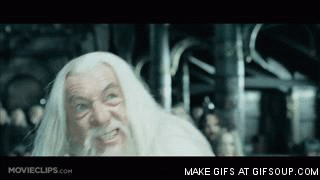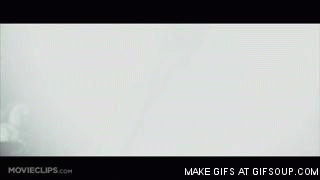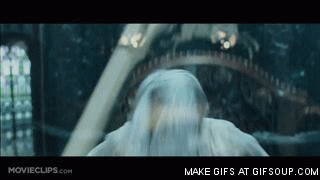Chimagination presentation
Chimagination is the mixture with agitation (with a magnetic bar of course) of cinema, imagination, and of course of Chemistry.
Summary
- Gandalf the White
- Roger Rabbit and the dip
- One Piece, ice and salt
- Arsenic pudding (2)
- The one ring !
- Catch them all !
- Arsenic pudding (1)
- Chloroformed Tintin
- That's not good ! That's nitroglycerin !
- Let the sky fall !
Gandalf the White
Gandalf the White, the Return of the King
- "Hahahaha, you have no power here Gandalf the gray" (Theoden in old schnock mode)
- (small sacred music "haaaaaaaa!")
- Hahaa! And bim in your face Sarouman, my clothes are of a brilliant whiteness (Gandalf)
- "Oh no, it's not possible! How does he get such white clothes ?!" (Saruman)
- And boom ! Stick hit !
That's the whole question, how does Gandalf go from gray to bright white? In the book it is stated that "it is sent back to earth because its task is not finished", but before this explanation a little idle the chemist sneers gently and a word comes to him in head: flavors !
White
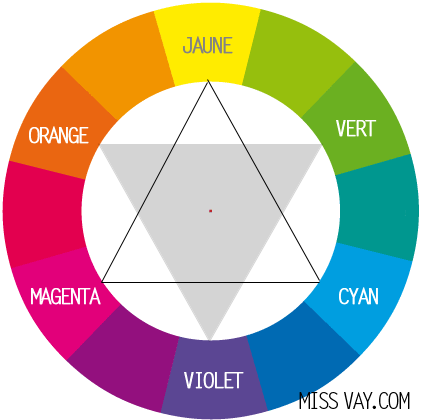
First of all let us specify what "white" is : a mixture of all colors. When the light arrives on an object, so that the latter appears to us white, it must therefore return all the wavelengths of the visible spectrum (hence all colors) to our eyes.
If an object absorbs a color of the visible spectrum, the complementary color appears. And if it absorbs all the colors it is black. We can therefore determine the colors that an object absorbs thanks to the chromatic circle.
The absorbed color is the opposite of the color that is observed :
"But tell me, Jamy, what connection with our dear friend Gandalf ?"
- Well it's very simple ...
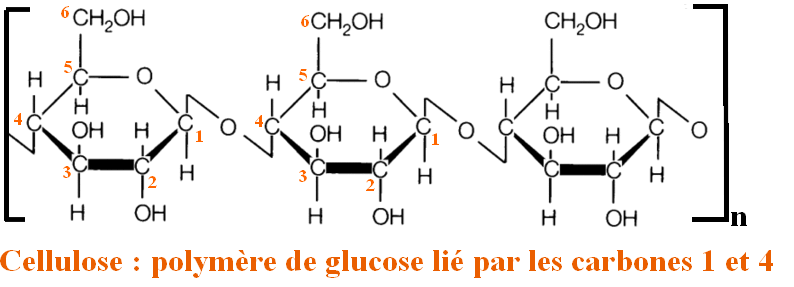
The concern our friend Gandalf should have is that his clothes are made of cotton. Cotton is composed essentially of cellulose : a polymer (repeat of the same chemical motif) of D-glucose.
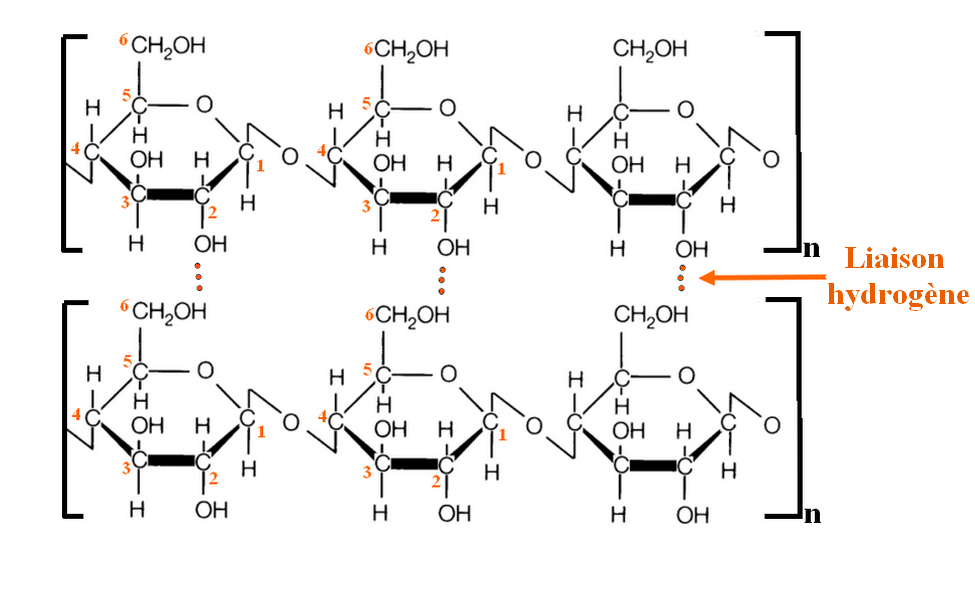
The fibers of cotton come from these long chains, linked together by hydrogen bonds (an electrostatic bond between a hydrogen and an oxygen here, a little less strong than a real covalent bond).
The main concern of cotton is that it absorbs in the blue, so normally all our clothes made of cotton should appear to us yellowish (complementary color of the purple-blue).
To overcome this problem, our friend Gandalf, really ahead of his time well taken care to add optical brighteners to his beautiful white cape.
The brighteners
The optical brighteners are compounds which are added to the garments (about 1% by weight), so that the latter appear white and not yellowish, or even "whiter than white".
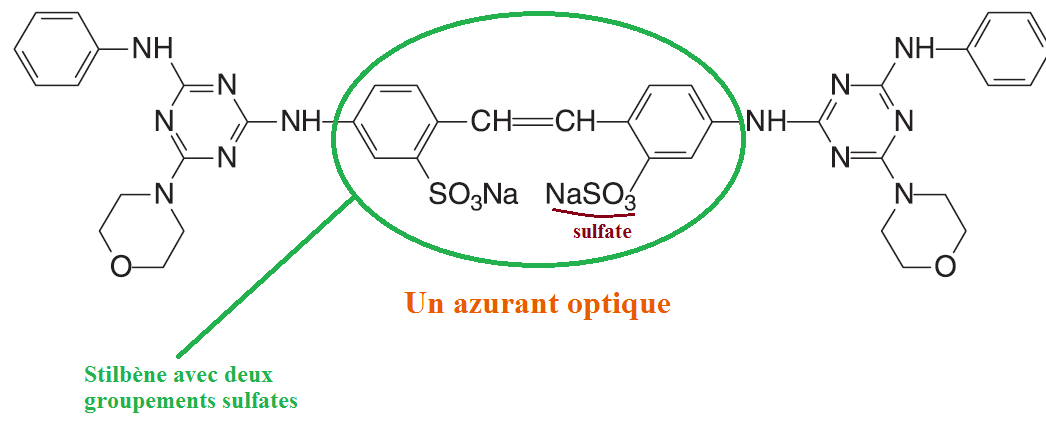
The brighteners are often stilbene derivatives and possess sulphate groups. These groups make the polar molecule and therefore ensure it a good solubility in water (which is important because the brighteners are widely used in detergents).
Moreover, these sulfate groups will allow the brightener to bind to the cellulose via hydrogen bonds (again and again). The bonds are made between the oxygens of the brightener (sulfate group) and the hydrogens of the alcohols of the cellulose.
The brightener has this ability to absorb light in the ultraviolet (it is therefore colorless). This absorption gives it an important energy: the molecule is then in an excited state and will thus be able to release its surplus energy in the form of heat or by emitting light: it is fluorescence!
The brighteners are therefore fluorescent molecules: they can therefore emit light and in particular blue light (around 400-500 nm), thus completing the missing color in the spectrum that the cellulose has absorbed.
But this is not all, for in addition to returning all the colors, the brightener emits light : hence this bright white, which is often called "whiter than white".
Laundry with optical brighteners
As a bonus, this is the famous sketch by Coluche, where he laughs at washing powders that wash "whiter than white" !
Bibliography
- Wikipedia for optical brighteners
- Wikipedia cellulose
- Articles of the chemical news on the laundry
Other articles
Roger Rabbit and the dip
Image of the film that wants the skin of Roger Rabbit
Hi everyone :)
Back to the 80's (and not the future;)) with the famous movie: "Who wants the skin of Roger Rabbit". In this film the Toons are alive and cohabit with humans. Roger Rabbit, the hero of the film is accused of the murder of Arvin Acme, director of the famous company ACME. He is then chased by the city judge who knows the only way to kill a Toon : the dip ! Let's study this chemistry dip to support :).
Here is an excerpt that explains what the dip is :
The dip is therefore the mixture of turpentine, benzene and acetone. But then :
- Are these compounds miscible?
- Are these compounds harmful? Corrosive?
- Do these compounds attack the plastic?
Turpentine
More precisely called turpentine, it is a colorless liquid with a characteristic pine odor. It is obtained by hydrodistillation (separation of an organic compound: here turpentine and an aqueous phase by heating and recondensation of vapors) of a pine resin such as maritime pine for example.
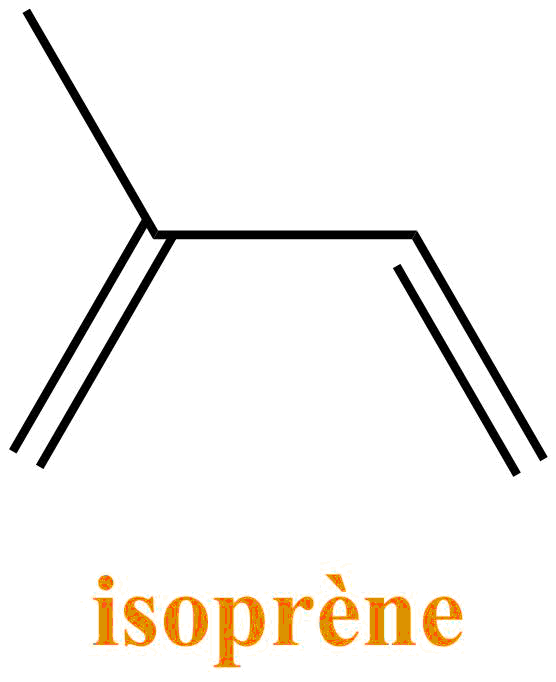
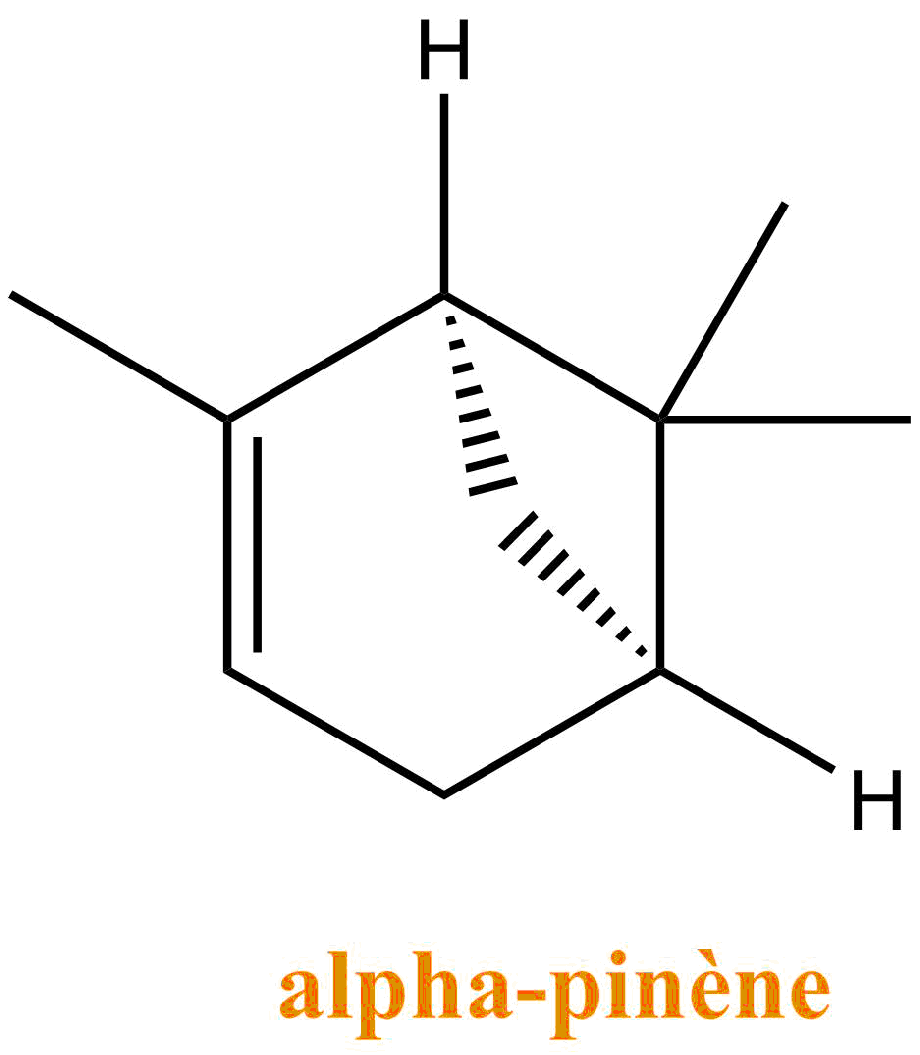
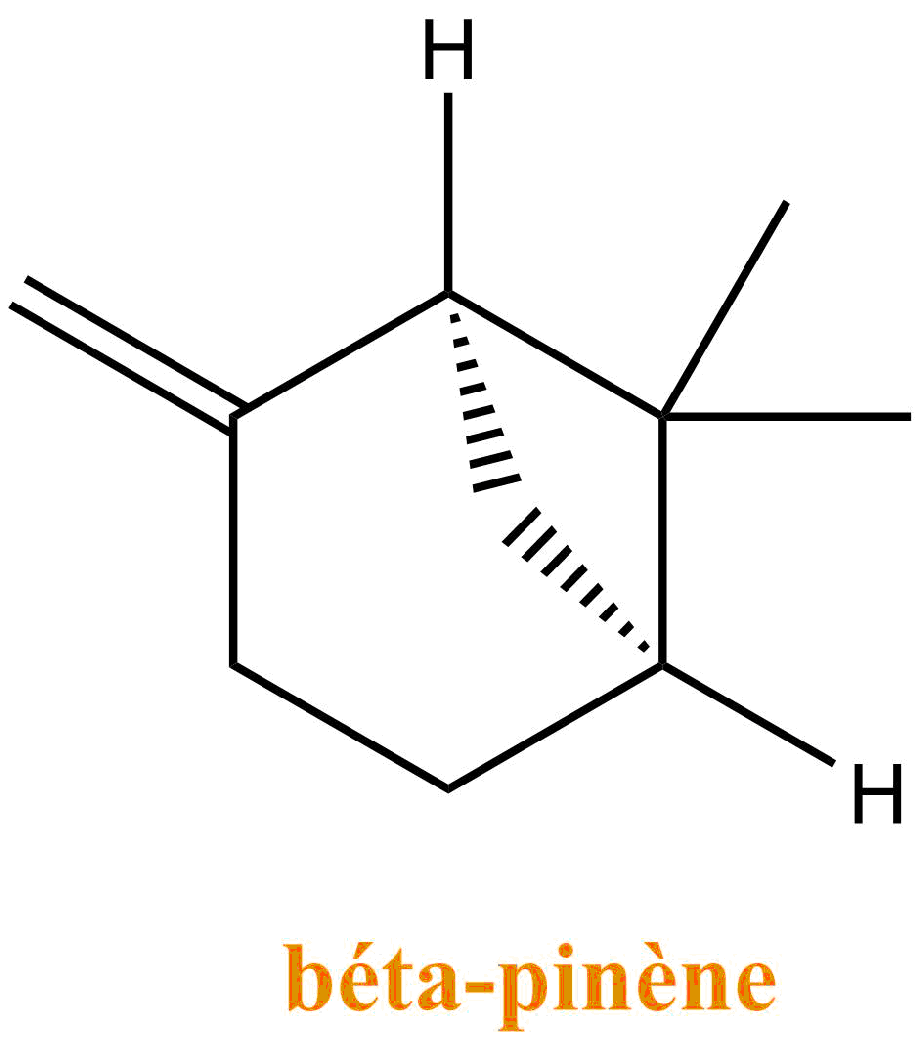
The turpentine consists of a mixture of terpenes: molecules resulting from the association of units with 5 carbons of isoprene type. They are the main constituents of essential oils of plants and flowers. Turpentine is mostly composed of α-pinene and β-pinene.
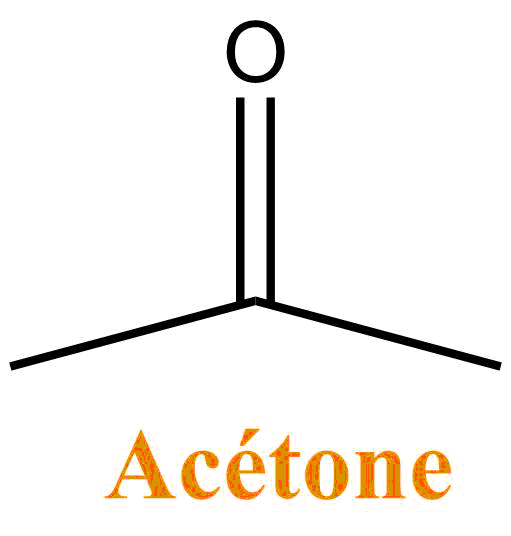
Acetone
It is a colorless liquid, volatile and widely used in organic chemistry as a solvent: it is especially miscible (forms a single phase) with water. Acetone is the main constituent of some solvents for removing nail polish. It is also used as a solvent for glues and paints.
Varnish remover Solvent bottle
(Painting, glue ...)
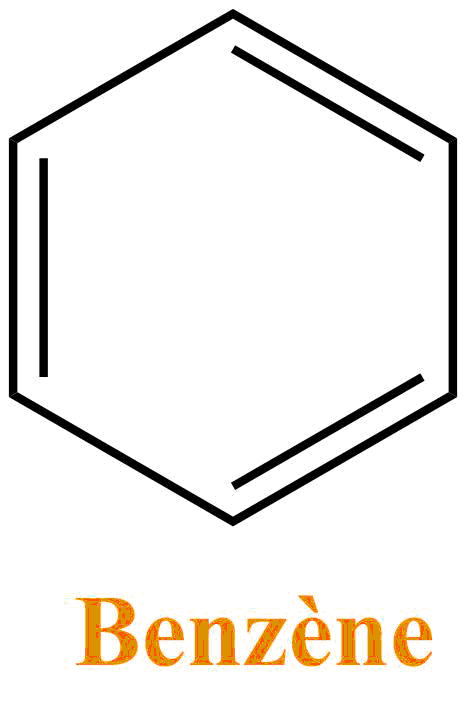
Benzene
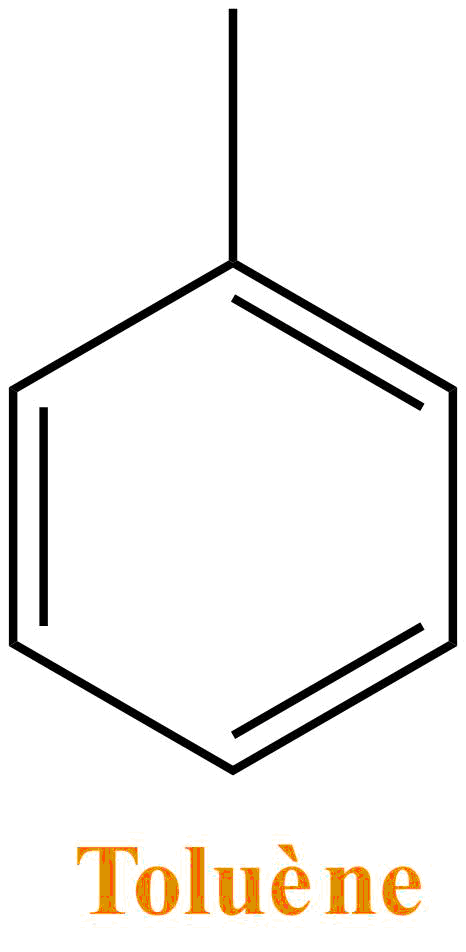
Benzene is a colorless liquid and is used as a solvent in organic chemistry even if its use is increasingly limited and replaced by toluene (a very similar structure).Benzene is not miscible with water and as many solvents it is very volatile. The sale and its use are highly regulated since it is known to be carcinogenic.
In Roger Rabbit
The appearance :
In the film the dip has a greenish color, but the turpentine, acetone and benzene are all three colorless, a coloring must surely be added by our dear Judge DeMort to make its substance more worrying. Turpentine, acetone and benzene are miscible with each other, so their mixture must a priori form a single phase.
It may also be noted that our three compounds are good solvents for organic molecules. Moreover, one of the characteristics of these three solvents is that they are volatile: that is to say they evaporate readily at ambient temperature. Let an acetone beaker stand and after a while the beaker will be empty.
This characteristic is more or less respected in the film, since we see vapors above the barrel, but the whitish appearance of these vapors would rather remind us of water vapor.
Paint solvent :
An organic solvent is often added to the paint in order to be able to liquefy it and to be able to spread it more simply. Once applied the paint will dry: the solvents present will then evaporate. Thus if an organic solvent is thrown onto a paint, the latter will dissolve in the solvent.
This is what is observed in the film when the judge dips the shoe into the dip, the latter sees its color dissolve in the barrel, thanks to the presence of our compounds sometimes used as a paint remover as we have Seen above.
Kill the Toons :
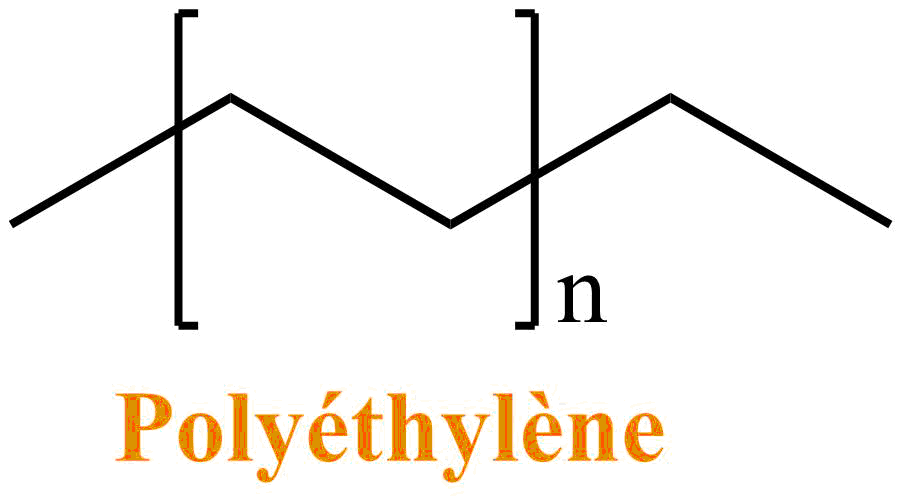
Suppose the Toons are made of plastic. Plastics are polymers: that is to say a regular assembly of the same molecular unit. The simplest and most used plastic is polyethylene (PE). It is present in many toys, but also in the plastic bags of supermarkets.
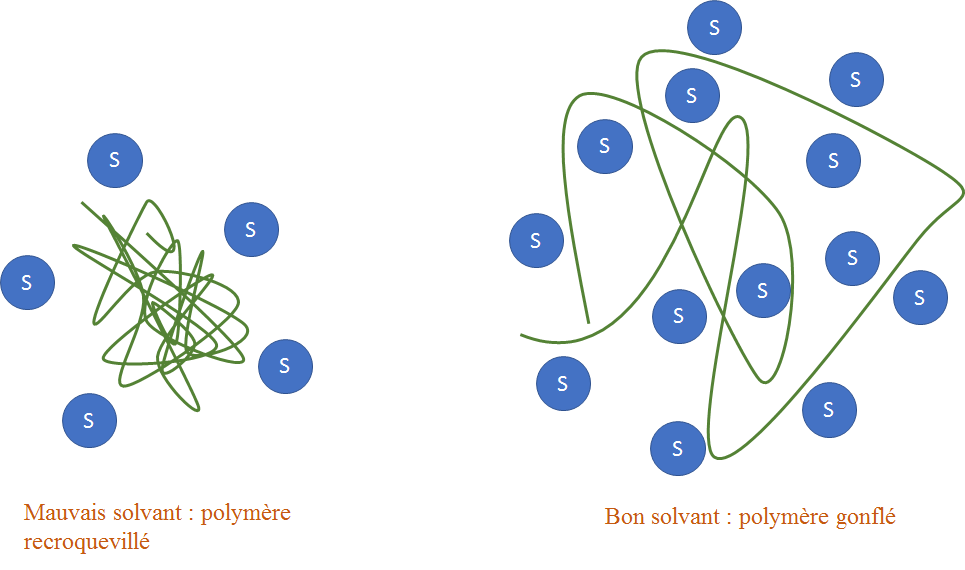
Some organic solvents can deteriorate plastics: such as benzene. Indeed, if the solvent interacts well with the polymer, the latter will "inflate" in order to favor the number of molecule of solvent in contact with its atoms: the polymer thus deteriorates.
If the solvent does not interact with the polymer, the polymer will remain curled up on itself (it is called a statistical ball) : it is not deteriorated.
Thus the dip would be lethal to the Toons, especially because of the benzene which would solvate the polyethylene well, and cause the Toon to deteriorate.
Bibliography
Other articles
One Piece, ice and salt
Hi everybody :)
While I was reading a chapter of the famous manga One Piece, I came across a phenomenon that immediately reminded me of the article on the "ice and salt challenge" that I published recently. For the curious article is available here: Ice and salt challenge
I remind you of the main lines of this article:
- We all know: salt + ice = melting ice
- But the mixture of salt and ice also reduces paradoxically the temperature of the ice
- Explanation: the salt ions want to be housed between the cohesion bonds of the ice (hydrogen bonds)
- Destruction of these connections requires energy taken from ice in the form of heat so its temperature decreases
In One Piece
The story takes place at scan 321 of One Piece or even at episode 228 for the animated ones. During this episode the crew of the straw hat meets one of the three general of the navy: Aokiji. The latter possesses the powers of a fruit of the demon: it has the ability to control the ice.
A struggle ensued between the crew of Luffy and General Aokiji. The difference in level between the pirates and Aokiji is very quickly felt and the latter finally freezes parts of the body of certain crew members, including Sanji and Zorro,
These last two, real warriors of the seas, do not wait their doctor and decide to treat their frozen arms themselves by throwing themselves in the sea!

One piece, scan 321 page 4 Oda Eiichiro

One piece, scan 321 page 5 Eiichiro Oda
Here is a video of the fighting scene, Sanji, Zorro and Luffy are freezing at 5 min.
What is the problem ?
As we have seen in the article "ice and salt challenge", the salt applied to ice in contact with the skin causes severe burns. Our two pirates throw themselves into the sea of ??salt water, so if it is true that the ice will melt more quickly than with fresh water, severe burns should be visible on their arms. But let's not forget that our two pirates are tough to cook;)
Bibliography
Other articles