Roger Rabbit and the dip
Image of the film that wants the skin of Roger Rabbit
Hi everyone :)
Back to the 80's (and not the future;)) with the famous movie: "Who wants the skin of Roger Rabbit". In this film the Toons are alive and cohabit with humans. Roger Rabbit, the hero of the film is accused of the murder of Arvin Acme, director of the famous company ACME. He is then chased by the city judge who knows the only way to kill a Toon : the dip ! Let's study this chemistry dip to support :).
Here is an excerpt that explains what the dip is :
The dip is therefore the mixture of turpentine, benzene and acetone. But then :
- Are these compounds miscible?
- Are these compounds harmful? Corrosive?
- Do these compounds attack the plastic?
Turpentine
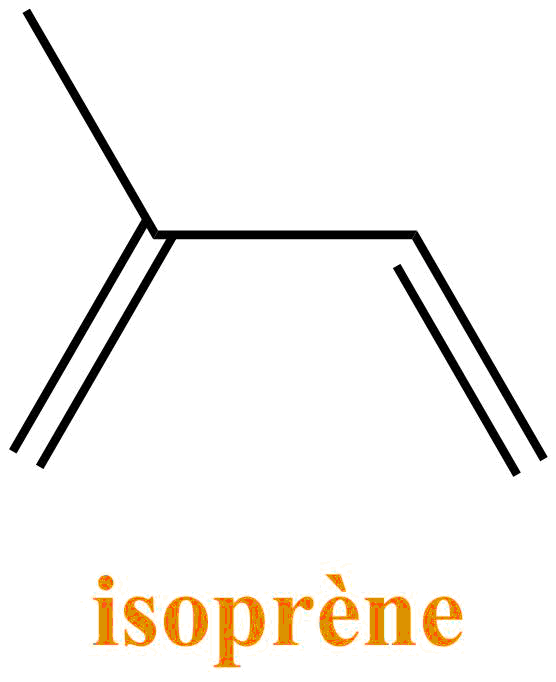
More precisely called turpentine, it is a colorless liquid with a characteristic pine odor. It is obtained by hydrodistillation (separation of an organic compound: here turpentine and an aqueous phase by heating and recondensation of vapors) of a pine resin such as maritime pine for example.
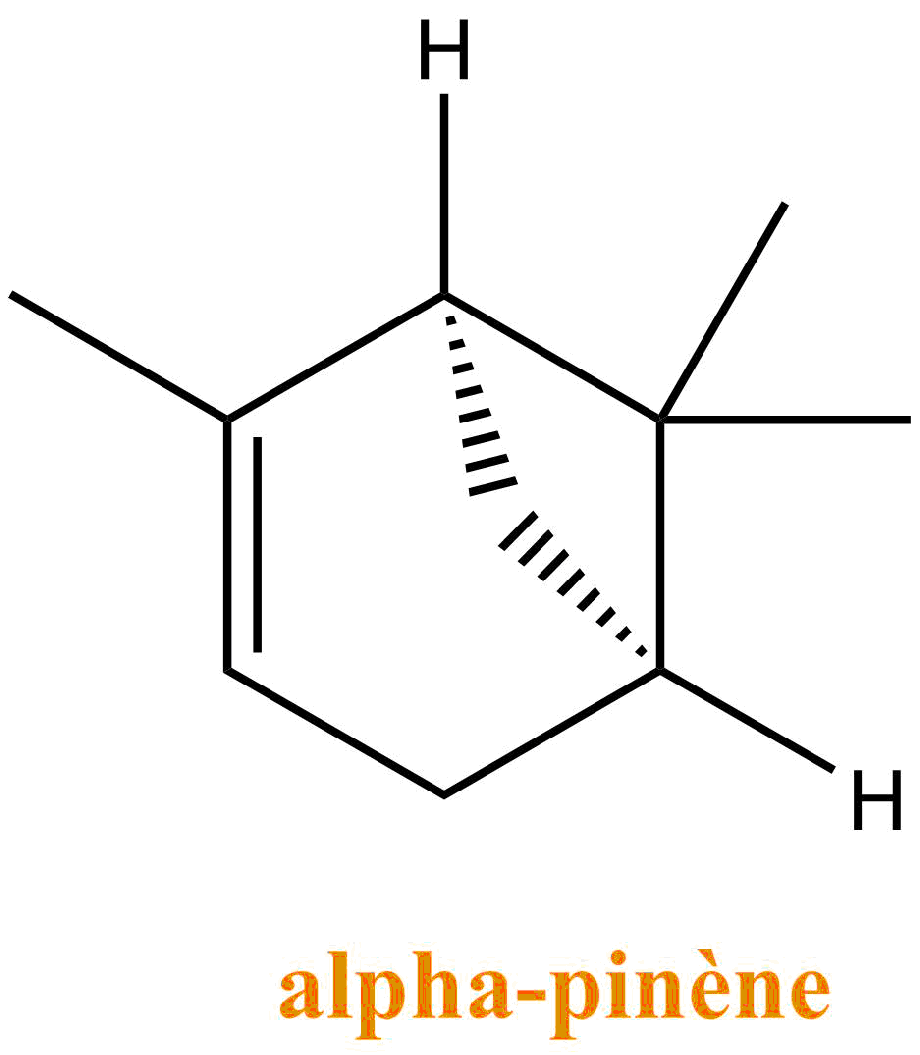
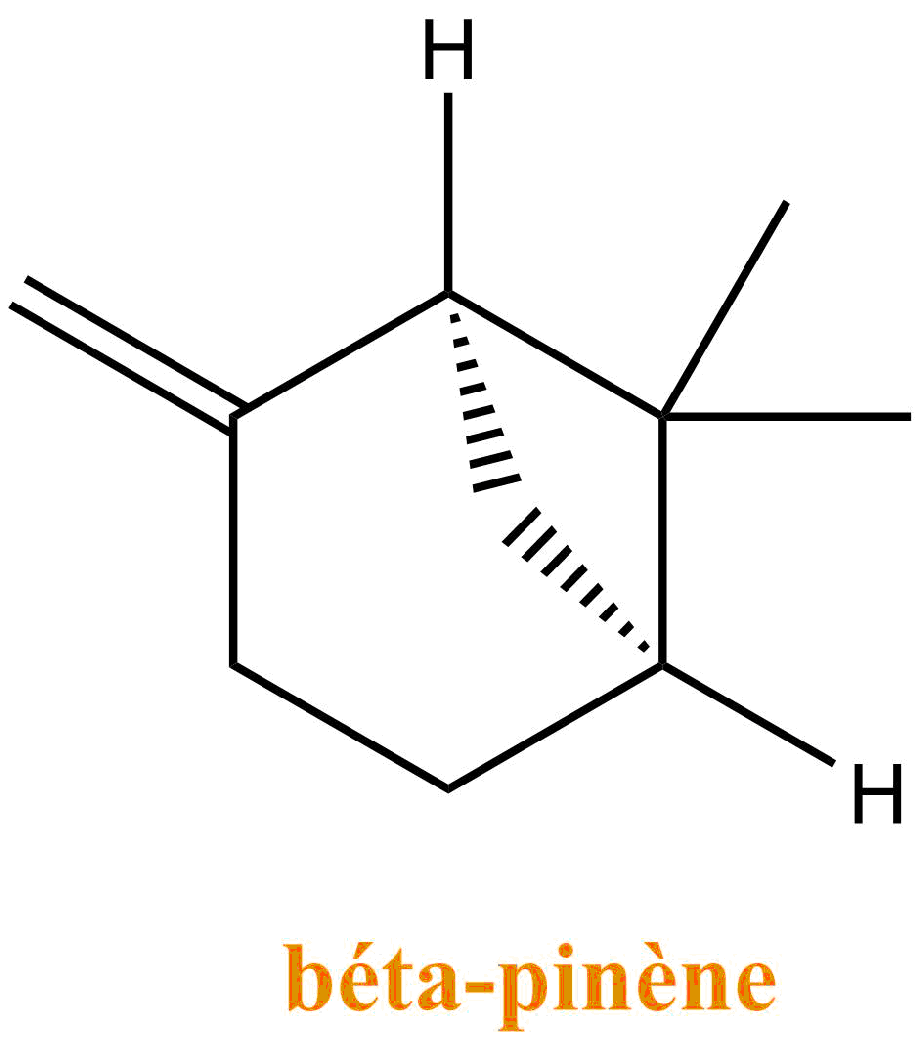
The turpentine consists of a mixture of terpenes: molecules resulting from the association of units with 5 carbons of isoprene type. They are the main constituents of essential oils of plants and flowers. Turpentine is mostly composed of α-pinene and β-pinene.
Acetone
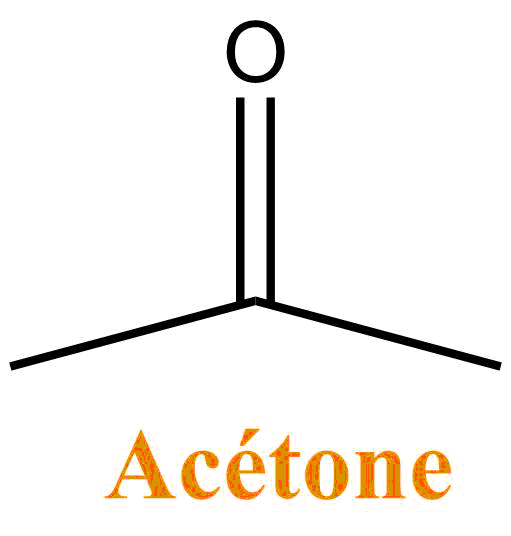
It is a colorless liquid, volatile and widely used in organic chemistry as a solvent: it is especially miscible (forms a single phase) with water. Acetone is the main constituent of some solvents for removing nail polish. It is also used as a solvent for glues and paints.
Varnish remover Solvent bottle
(Painting, glue ...)
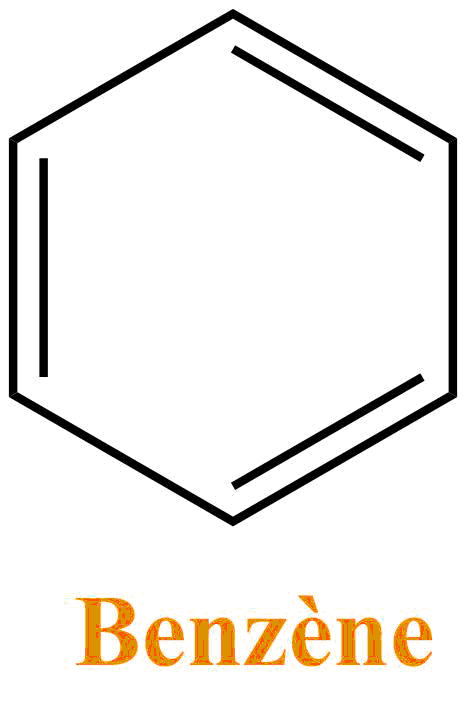
Benzene
Benzene is a colorless liquid and is used as a solvent in organic chemistry even if its use is increasingly limited and replaced by toluene (a very similar structure).Benzene is not miscible with water and as many solvents it is very volatile. The sale and its use are highly regulated since it is known to be carcinogenic.
In Roger Rabbit
The appearance :
In the film the dip has a greenish color, but the turpentine, acetone and benzene are all three colorless, a coloring must surely be added by our dear Judge DeMort to make its substance more worrying. Turpentine, acetone and benzene are miscible with each other, so their mixture must a priori form a single phase.
It may also be noted that our three compounds are good solvents for organic molecules. Moreover, one of the characteristics of these three solvents is that they are volatile: that is to say they evaporate readily at ambient temperature. Let an acetone beaker stand and after a while the beaker will be empty.
This characteristic is more or less respected in the film, since we see vapors above the barrel, but the whitish appearance of these vapors would rather remind us of water vapor.
Paint solvent :
An organic solvent is often added to the paint in order to be able to liquefy it and to be able to spread it more simply. Once applied the paint will dry: the solvents present will then evaporate. Thus if an organic solvent is thrown onto a paint, the latter will dissolve in the solvent.
This is what is observed in the film when the judge dips the shoe into the dip, the latter sees its color dissolve in the barrel, thanks to the presence of our compounds sometimes used as a paint remover as we have Seen above.
Kill the Toons :
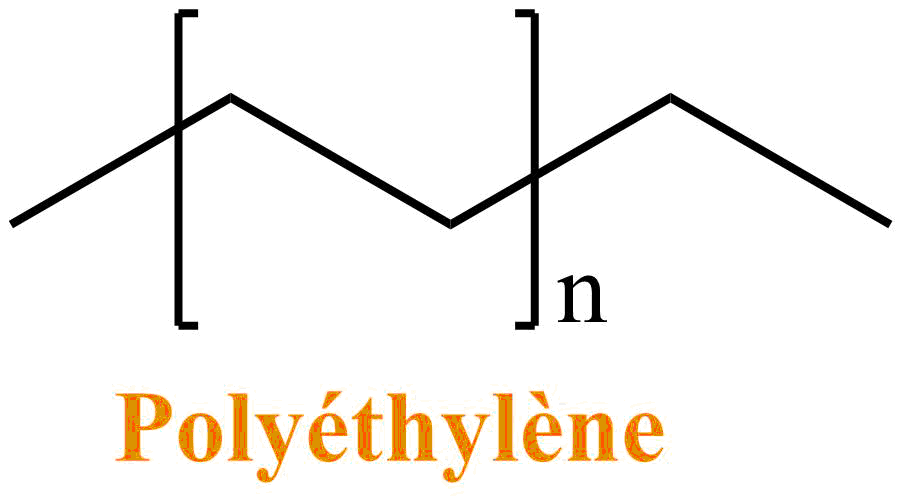
Suppose the Toons are made of plastic. Plastics are polymers: that is to say a regular assembly of the same molecular unit. The simplest and most used plastic is polyethylene (PE). It is present in many toys, but also in the plastic bags of supermarkets.
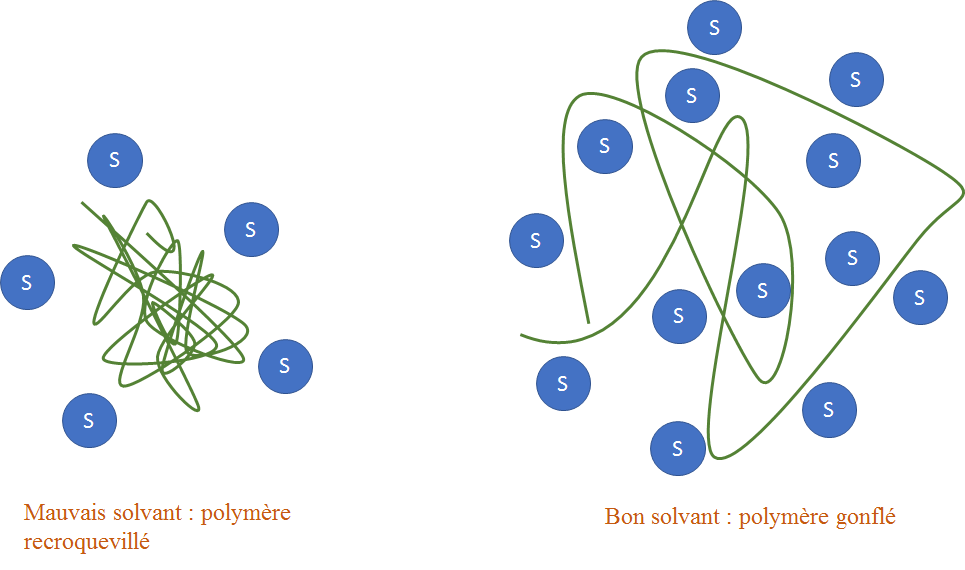
Some organic solvents can deteriorate plastics: such as benzene. Indeed, if the solvent interacts well with the polymer, the latter will "inflate" in order to favor the number of molecule of solvent in contact with its atoms: the polymer thus deteriorates.
If the solvent does not interact with the polymer, the polymer will remain curled up on itself (it is called a statistical ball) : it is not deteriorated.
Thus the dip would be lethal to the Toons, especially because of the benzene which would solvate the polyethylene well, and cause the Toon to deteriorate.
Bibliography
Other articles
Inscrivez-vous au blog
Soyez prévenu par email des prochaines mises à jour
Rejoignez les 13 autres membres