Catch them all !
Hello everyone :)
"It takes courage, Poookééémooon !!! Catch them all ! Catch them all ! What better way to give yourself the potato than this splendid generic Pokémon! The world of Pokémon is very extensive and very detailed, and this is what all scientific research requires: data to be studied.
And to study this vast universe all good trainer must have an indispensable tool: the pokedex! The latter gives us very interesting information about Pokémon, sometimes even suggesting scientific phenomena. In this article we will study Fantominus, chemistry in support!
The possible calculations are available via orange clickable links arranged in the appropriate places, do not hesitate to take a look ;)
Gastly
Poképedia.fr
What the pokedex teaches us :
- It is 1.3 m in diameter for 0.1 kg
- A poisonous gas composed 95% of its body: causing fainting and suffocations
- It often appears in black, spherical, surrounded by violet mist
- It has a sweet smell
Therefore, we can ask many questions about Fantominus: what kind of gas is it composed? How does he fly? How does he stifle his victims?
What are the compatible gases ?
Suppose that Fantominus is 100% gas.
We can therefore determine the density of Fantominus, that is to say the weight it weighs by volume of gas. This density can help us determine the component gas Fantominus. Indeed there are tables listing the orders of magnitude of the densities of the gases.
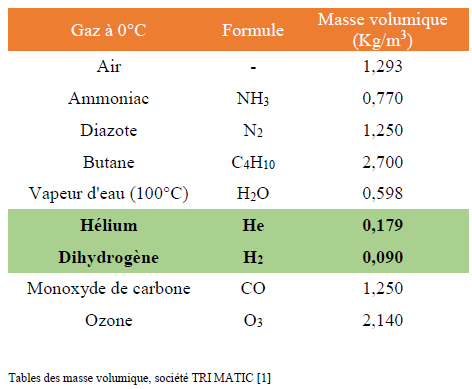
The gas that composes Fantominus has a density of ρ F = 0.0870 kg / m 3 .
To get an idea, gaseous air, composed mainly of N 2 diazote, has a density of 1.2 kg / m 3 . Thus we need gases composed of very light elements in order to have a much lower density.
The lightest elements of the periodic table are hydrogen H and helium He.Hydrogen gas is more stable in the form of H 2 dihydrogen and helium is gaseous at ambient temperature and pressure. Their densities are given in the table below, determined at 0 ° C. and at atmospheric pressure. [1]
H 2 appears to be a good candidate, however the indicated density corresponds to a temperature of 0 ° C. The temperature at which to heat our gas can be Ρ F =0.0870 kg / m 3 .
Indeed, when a gas is heated, it becomes less dense, "lighter" in a way. This is the principle used by balloons.
The relationship between the density of a gas and its temperature can be determined by the coefficient of expansion of the gas. Indeed, the more a gas is heated (at constant pressure), the more the particles of this gas will be stirred and thus occupy a larger volume, the volume being larger the density decreases therefore. It is the expansion of the gas under the effect of temperature.
With helium component Fantomius it would be necessary to heat the latter to 141 ° C in order to obtain ρ F = 0,0870 kg / m 3 .
With dihydrogen, it would be necessary to heat Fantominus at 9.1 ° C.
Detail-calculation-temperature
If we assume that Fantomius is at the same temperature as the outside temperature, then H 2 seems the most appropriate gas.
Is H 2 compatible with other pokédex information ?
Dihydrogen can effectively lead to suffocation but only for lack of dioxygen.Fantominus could therefore stifle his opponents by wrapping them around. However the latter is not really toxic.
Moreover, this gas is not of violet color and does not have a sweet odor as specified in pokédex.
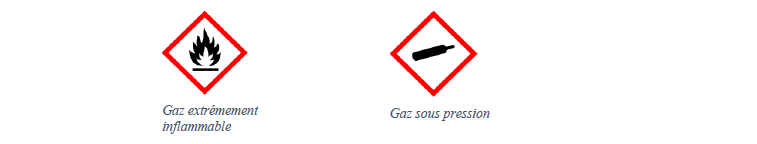
Finally H 2 is an extremely flammable gas, it is very sensitive to any source of flames, heat, sparks. If Fantominus was truly made up of dihydrogen one could assume that he would have a certain sensitivity (resistance or weakness) towards fire and electric pokémons, which is not the case.
One could imagine, for example, that it would ignite after a fire attack and would thus become more powerful.
H 2 was used in airships, and is suspected of being responsible for the Hindenburg fire (airship using H 2 ).
Hazard symbols for H 2 :
How and at what altitude does Fantominus fly ?
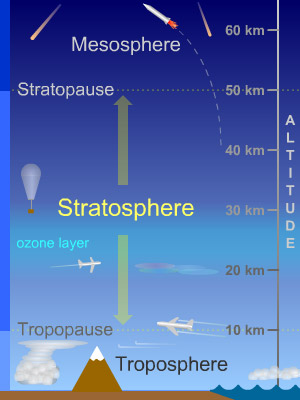
We note that we have a problem with the density that we obtain for Fantominus: ρF = 0.0870 kg / m 3 . Indeed according to this last Fantominus should float at a constant altitude of about 20 km, which is enormous, it is 4 times the height of Everest. At this height, difficult to capture one;)
Credit: Randy Russell, UCAR
To determine the altitude of Fantominus, it is sufficient to find the altitude for which the density of the air is equal to the density of Fantominus. Indeed as long as Fantominus is less dense than the air, it climbs, and as soon as it is denser it descends. It is the competition between the force of gravitational attraction of the Earth and the thrust of Archimedes exerted by the air. The density of the air varies according to the altitude, so the height for which the density of the air is equal to that of Fantominus: 20 km [2] can be determined.
There is therefore a problem with the density found for Fantominus, and therefore with its mass since the latter seems to have a correct volume (if we refer to the cartoon). Another possibility would be that Fantominus exerts himself an additional force preventing him from flying.
Other possible gases
One of the few violet compounds is the diode, however the latter is liquid at ambient temperature and pressure. Thus one can envisage that Fantominus consists of a mixture of a toxic gas not too dense to be able to fly (as NH 3 ) and of diode to allow this purple color.
References
- Wikipedia
- Poképédia for information about Fantominus
- Http://cfppah.free.fr/docs/Table_des_masses_volumiques.pdf [1]
- Http://www.planete-sciences.org/espace/IMG/pdf/characteristiques-atmosphere_meca-vol-ballon.pdf , page 4 [2]
Other articles
Inscrivez-vous au blog
Soyez prévenu par email des prochaines mises à jour
Rejoignez les 13 autres membres