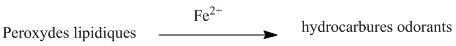Money has no smell
Asterix and the cauldron, A. Uderzo
- Smell !
- Smelling money ? But it does not smell !
Everybody knows this famous expression: "the money has no smell", it is the Roman emperor Vespasian who would have proclaimed it towards -70 before JC in order to justify the implementation of a tax On the collection of urine.
Yet a characteristic odor is evident from our coins when we handle them, an odor often called "metallic". What, then, is this odor? And where does she come from? These questions were answered by German scientists in one of their publications in 2006. (" The Two Odors of Iron")
Sweat Corrodes Metal
In order for an odor to be perceptible, the molecule responsible for this odor must be volatile, which is not the case with iron and other metals present in coins. The origin of the phenomenon comes from the action of sweat on iron. Indeed when we handle them, the pieces soak up a bit of sweat that we have along our skin. The sweat is slightly acidic (pH = 4 to 6), and will thus cause the oxidation of iron in Fe 2+ ion.
In fact, irrespective of the pH, the iron is not stable in an aqueous medium and, in an acid medium, the latter will in particular oxidize to iron ion II. It is the same phenomenon that is responsible for the corrosion of iron and steel (pipelines, boat hulls ...).
Oxidation of iron in aqueous medium
Reduction of lipid peroxides of the skin
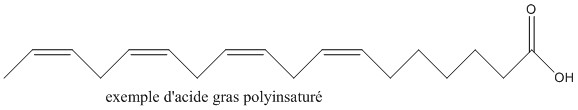
Our skin is made up of fat, specifically polyunsaturated fatty acids. They are carboxylic acids with a very long carbon chain and possess a number of unsaturations (double bonds).
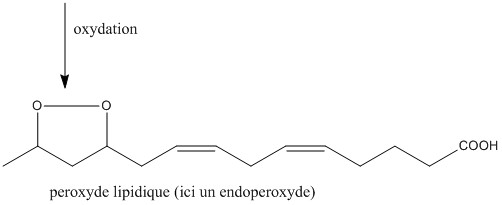
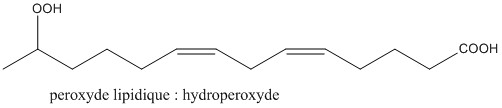
The latter are liable to oxidize to lipid peroxides by the action of enzymes (lipoxygenase), or else by oxidative aggression. Oxidative aggression is defined by the presence of a large quantity of radical species in the organism, with a high oxidizing power. These can come from various mechanisms (respiration in the mitochondria, action of the UV ...).
or :
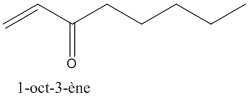
Finally, these lipoperoxides will be reduced by the Fe 2+ ions formed by the sweat. There is then formation of odoriferous carbonylated hydrocarbons such as 1-oct-3-ene, which is the compound that is mainly responsible for the "metallic" smell of coins. This last reaction is very rapid, hence the illusion that the odor comes from metal.
References
Other articles
Inscrivez-vous au blog
Soyez prévenu par email des prochaines mises à jour
Rejoignez les 13 autres membres