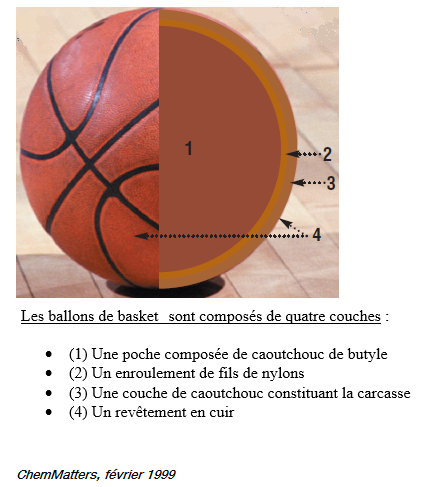
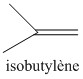Basketball
The chemistry of the basketball (2)
Here is the continuation of the composition of our favorite ball :)
Here is the link of the first part for possible reminders
2. The second layer
Once the first layer of butyl rubber has been obtained, the second layer is logically passed to the second layer.
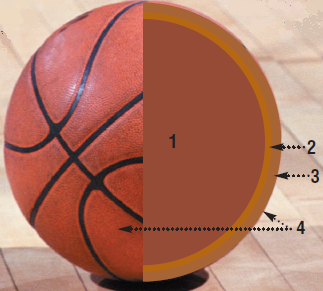
Reminder of the appearance of the first layer:
alibaba.com
We now surround our spherical pocket with nylon or polyester threads. These are strong twines that will maintain the shape of the basketball, and in particular to avoid the formation of bumps. Balloons of high quality can have more than 3000 m of wire. The polyester is certainly less expensive for manufacturers but also less resistant than nylon: it will give less durable balloons.
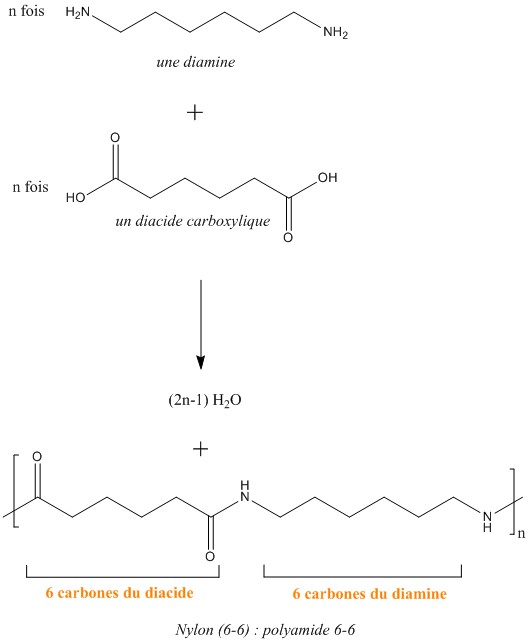
Synthesis of nylon:
The nylon is a copolymer of a dicarboxylic acid and a diamine, then a polyamide is formed.This is a stepwise polymerization reaction: this means that the monomers react with one another to form dimers, then trimers, and thus to the polymer. More precisely, polycondensation (removal of one molecule of water at each step) is present here.
Above is the synthesis of nylon 6-6, but there are other types such as 4-6 or 2-6 (the first digit indicates the carbon number of the diamine). Nylon has been greatly exploited by the textile industry especially by creating stockings and tights, it is also widely used for sporting and molded articles, and also for fishing yarns. These farms are largely linked to its ultra-resistance, for example for a monofilament fishing line the breaking strength is more than 100kg.
Old pub for nylon stockings:
lesechos.fr
0.8 mm nylon thread:
alittlemercerie.com
On the internet you will be able to easily find videos of synthetic nylon, here are two:
Back to our basketball, we had the option of using nylon or polyester. The polyester is also a polymer composed of the repetition of an ester function. The reaction is very similar to that of nylon and this time takes place between a dicarboxylic acid and a diol (molecule with two alcohol functions).
Third layer of balloon
The third layer covering the other two is made of rubber (natural, composite or butyl rubber).This stage is realized in a mold in order to make appear the external reliefs on the balloon (furrows, logos ..). The color and stickers are added into the mold just before vulcanizing the rubber. The high-quality balloons even have a leather covering over them.
References
- Article ChemMatter, February 1999, "The chemistry of basketball"
- Wikipedia
The chemistry of the basketball (first part)
Being a big fan of basketball, I can not help but write my first article on the chemistry of the basketball. And what to say about this indispensable equipment! The requirements of the federations are very strict and precise on the quality of the balloon, so the choice of materials and manufacturing processes are reflected accordingly. So here's a quick summary of the WNBA rules about the basketball:
- Perfectly spherical, correct circumference
- Being wear-resistant
- Waterproof valve and balloon
- Rebound correctly
- Ball not too heavy (about 600 g)
The composition of a basketball
1. The inner pocket
The inner spherical pouch must be completely impermeable to gases, especially those present in the air (O2, N2) in order for the balloon to remain properly inflated. But this pocket must also be elastic and resistant enough.
The simple rubber if it meets the two previous requirements, is however not sufficiently gas-tight. Thus it is a material close to the latter which is used: butyl rubber. This elastic polymer is very famous for its excellent gas impermeability, it is used especially in the industry of tires, inner tubes, industrial joints.
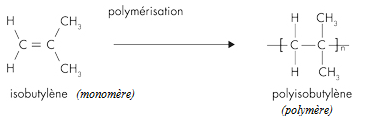
Before explaining the structure of butyl rubber, it is important to understand what a polymer is.A polymer consists of macromolecules. The latter are molecules of very large molecular mass, and characterized by the repetition of small molecules: the monomers.
Examples of natural polymers:
- The rubber
- Proteins (polymers of amino acids)
- DNA
- Starch
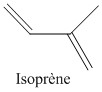
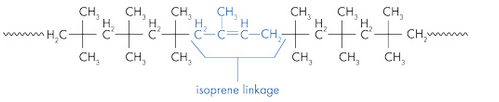
Butyl rubber is a copolymer of isobutylene and isoprene, which means that its structure is constituted by the repetition of these two monomers. The butyl rubber is in fact mainly composed of isobutylene and contains little isoprene (about 2% for a chain).
Initially the material contained only isobutylene, polyisobutylene (PIB) was then obtained, and then, to give it elastic properties (rubber), a small amount of isoprene was added. The latter will make it possible to introduce C = C double bonds into the polymer.
www.techniques-ingénieur.fr
Structure of butyl rubber:
www.techniques-ingénieur.fr
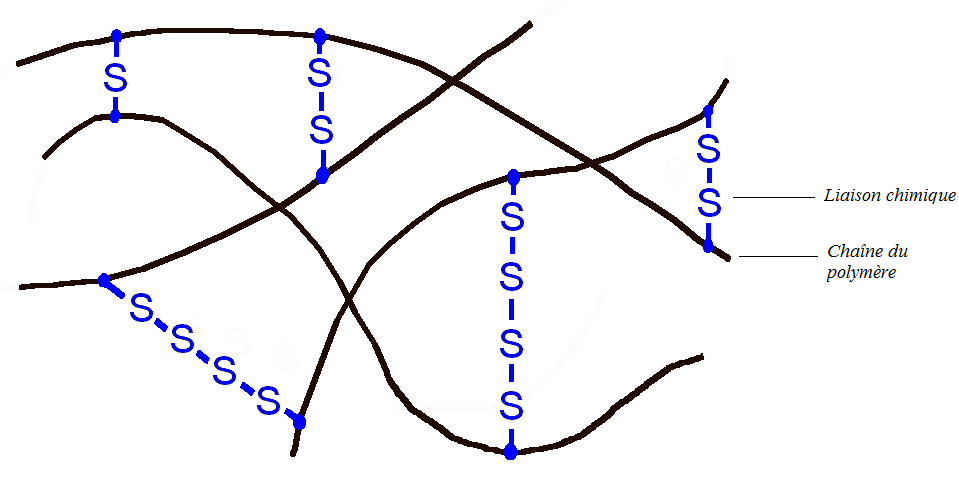
The presence of the C = C double bond is very important, in fact a reactive functional group has been introduced into the polymer. The objective is then to connect each polymer chain by chemical bonds, which is possible thanks to the double bonds. This process is carried out at high temperature and in the presence of sulfur (vulcanizing agent): this is vulcanization.
Vulcanization of butyl rubber
Wikipedia
The material then becomes much more elastic, it is an elastomer. The latter can then be stretched up to twice its original size and return very quickly to its original shape: this is the origin of the rebound of the basketball. Another interesting feature is that the material consists of only one giant molecule. Thus the different parts of the molecules can no longer move with respect to each other whatever the temperature. The material can no longer be melted (liquid state). So if you burn a basketball, little by little the latter will carbonize without going through a liquid state. (Note: Black fumes from combustion are toxic and carcinogenic).
This last point demonstrates that it is important to give the spherical shape well to our butyl rubber just before the vulcanization of the material, because it is then impossible to melt our material to give it the desired shape.
First layer of basketball made of butyl rubber
alibaba.com
Example of gloves made of butyl rubber

protective.ansell.com
Video where you burn a basketball
Warning ! Important hazards associated with such an experiment: handling of the fire, risks of fire, major burns, toxicity of smoke ...
The second part of this article will be dealt with next week with the program: layers 2,3,4 as well as the synthesis and properties of nylon. Good week :)
References
- ChemMatter article, February 1999, "The chemistry of basketball"
- www.techniques-ingenieur.fr