The chemistry of the basketball (2)
Here is the continuation of the composition of our favorite ball :)
Here is the link of the first part for possible reminders
2. The second layer
Once the first layer of butyl rubber has been obtained, the second layer is logically passed to the second layer.
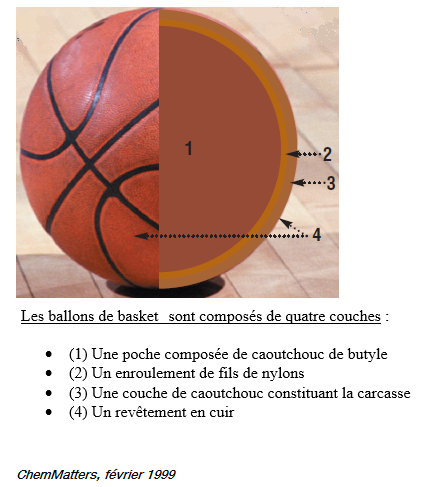
Reminder of the appearance of the first layer:
alibaba.com
We now surround our spherical pocket with nylon or polyester threads. These are strong twines that will maintain the shape of the basketball, and in particular to avoid the formation of bumps. Balloons of high quality can have more than 3000 m of wire. The polyester is certainly less expensive for manufacturers but also less resistant than nylon: it will give less durable balloons.
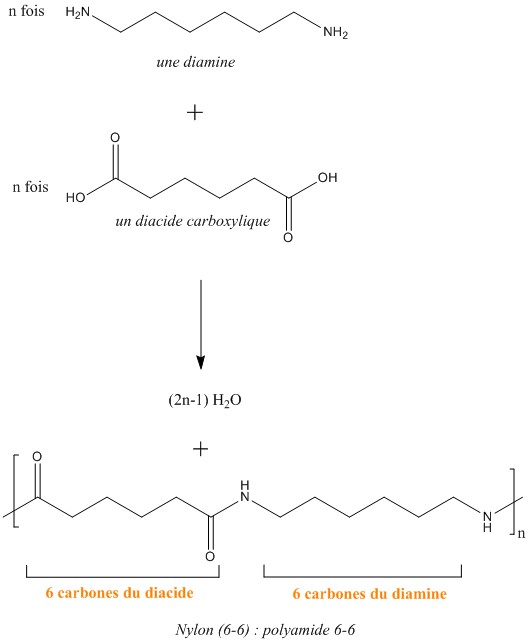
Synthesis of nylon:
The nylon is a copolymer of a dicarboxylic acid and a diamine, then a polyamide is formed.This is a stepwise polymerization reaction: this means that the monomers react with one another to form dimers, then trimers, and thus to the polymer. More precisely, polycondensation (removal of one molecule of water at each step) is present here.
Above is the synthesis of nylon 6-6, but there are other types such as 4-6 or 2-6 (the first digit indicates the carbon number of the diamine). Nylon has been greatly exploited by the textile industry especially by creating stockings and tights, it is also widely used for sporting and molded articles, and also for fishing yarns. These farms are largely linked to its ultra-resistance, for example for a monofilament fishing line the breaking strength is more than 100kg.
Old pub for nylon stockings:
lesechos.fr
0.8 mm nylon thread:
alittlemercerie.com
On the internet you will be able to easily find videos of synthetic nylon, here are two:
Back to our basketball, we had the option of using nylon or polyester. The polyester is also a polymer composed of the repetition of an ester function. The reaction is very similar to that of nylon and this time takes place between a dicarboxylic acid and a diol (molecule with two alcohol functions).
Third layer of balloon
The third layer covering the other two is made of rubber (natural, composite or butyl rubber).This stage is realized in a mold in order to make appear the external reliefs on the balloon (furrows, logos ..). The color and stickers are added into the mold just before vulcanizing the rubber. The high-quality balloons even have a leather covering over them.
References
- Article ChemMatter, February 1999, "The chemistry of basketball"
- Wikipedia
Inscrivez-vous au blog
Soyez prévenu par email des prochaines mises à jour
Rejoignez les 13 autres membres