Ice and salt challenge
(Photo: @gendarmerie nationale)
A challenge has returned to fashion on social networks : the "ice and salt challenge". The latter consists of applying salt to the skin and then adding ice over it. Very quickly a burning sensation is felt, the objective of the challenge being to hold as long as possible.
But then how can such common ingredients provoke such severe burns ? Let's analyze the phenomenon, chemistry in support !
observations
Small experience made in small beakers;)
explanations
In order to understand this phenomenon, we first have to look at the structure of water.
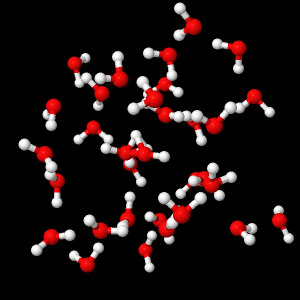
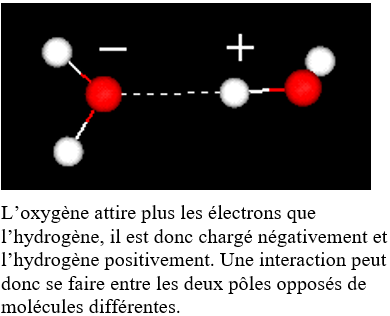
Liquid water consists of a very large number of water molecules (5 to 6 moles of water for one glass of water, thus approximately 10 24 H 2 O molecules) which establish electrostatic links between them ( Called hydrogen bonds). These bonds are constantly made and disassembled, and each molecule is sufficiently free of its motion.
Arrangement of water molecules in liquid phase
Hydrogen bonding between two water molecules
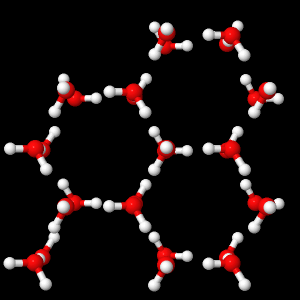
In the solid phase, the molecules are much more fixed, the water molecules remain close to a position of equilibrium. They form lasting hydrogen bonds between them and the structure obtained is symmetrical.
Solid phase water molecules (ice)
Water passes from the liquid to solid state at 0 ° C: it is solidification. The inverse action, when melting ice is fusion.
Now what happens when salt is brought into contact with ice ?
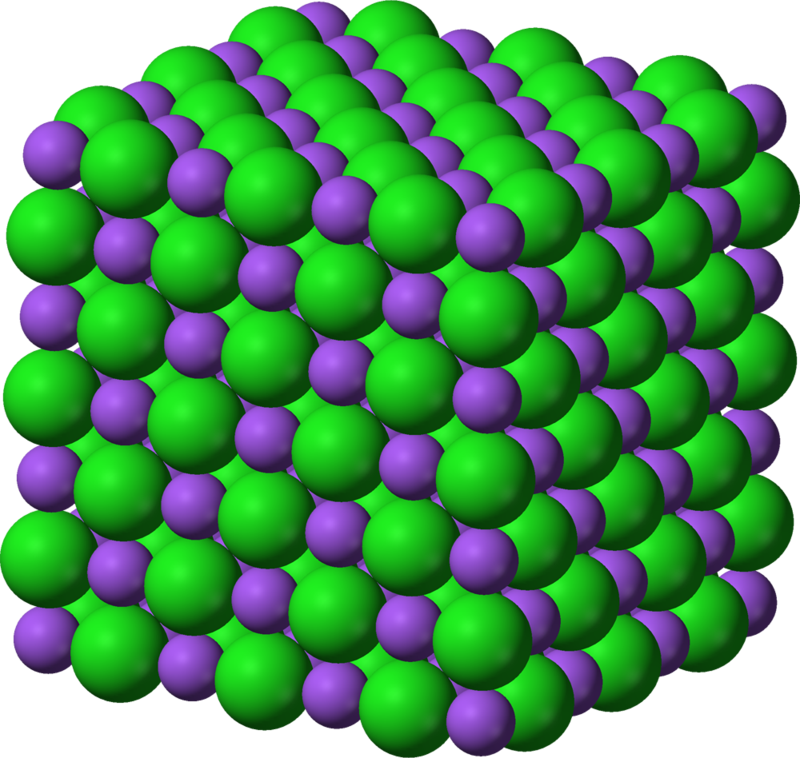
The solid salt also has a well organized and very symmetrical structure. The salt consists of sodium Na + ions (in violet in the figure) and chloride ions Cl - (in green in the figure), and its crude formula is NaCl (s).
Solid structure of the salt : NaCl (s)
wikipedia
The bonds between the Na + and Cl - ions are ionic in nature (bonds between a cation and anion).
For the following it is important to understand that breaking a chemical bond requires energy and building a bond provides energy.
To understand this we can imagine two lovers kissing (# Metaphor Valentine), try to separate them : it will be necessary to put in his : therefore energy. Conversely, when one assembles them, one will run to the other and the "shock" of their encounter will release energy.
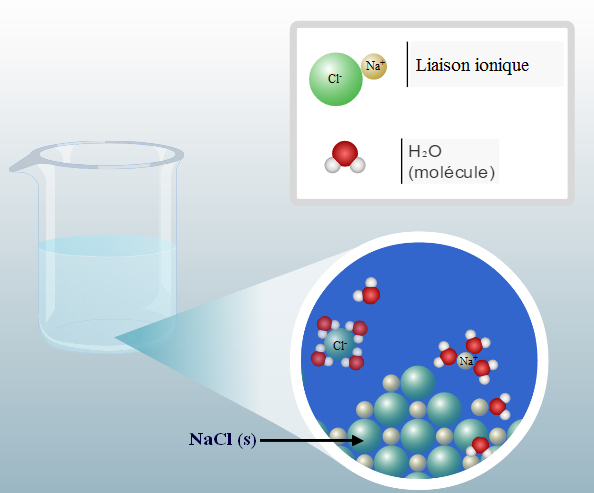
When salt is deposited on an ice cube, the latter has a thin film of liquid water on its surface, the NaCl salt (s) will dissolve in this water film. This dissolution does not require energy and neither does it provide: this phenomenon is said to be athermic.
Dissolution and solvation of NaCl with H 2 O
www.edumedia-sciences.com
Then the solvated ions will come into contact with the solid ice, they will then desire to insert between the hydrogen bonds and to tear the molecules of water with the ice. This phenomenon will increase the solvation of cations and anions.
However, this process breaks down hydrogen bonds between water molecules, and for this it takes energy (endothermic process). This energy will come from the ice in the form of heat.The ice will provide heat to allow solvation of the ions.
The consequences of all this are the reduction of the temperature of the ice (loss of heat) and the melting of the latter since the hydrogen bonds which break the solid ice were broken.
And here is the paradox: the process decreases the temperature of the ice and melts at the same time!
The temperature of the ice can thus drop to -21.6 ° C and cause severe burns from the cold.To be more precise, water is said to form an eutectic mixture with salt. It is the mixture of two pure bodies which melts and solidifies at constant temperature.
Bibliography
- https://fr.wikipedia.org/wiki/Chlorure_de_sodium
- https://fr.wikipedia.org/wiki/Eutectique
- http://phymain.unisciel.fr/du-sel-pour-refroidir/
Other articles
Inscrivez-vous au blog
Soyez prévenu par email des prochaines mises à jour
Rejoignez les 13 autres membres