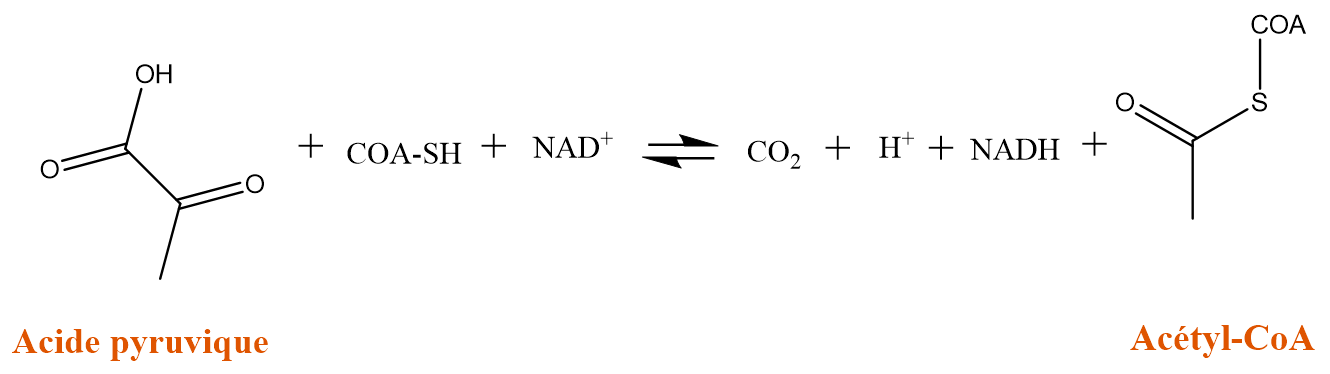Arsenic pudding (2)
Hello everyone :)
Here is the continuation of the study of the ingredients of our famous pudding with the Arsenic. Today we will study Arsenic precisely!
Link to the first article: Arsenic pudding (1)
Arsenic
Arsenic with atomic number 33 and chemical symbol As is a rather abundant compound on Earth (51th element of the most abundant table). More than 99% of the arsenic present in the Earth's crust is in the form of ore. In the atmosphere arsenic comes from the production industries of As 2 O 3 : arsenic trioxide, the latter being mainly used to bleach glass. Finally, volcanic activity or forest fires may be responsible for natural emissions of arsenic.
All the compounds of Arsenic are very toxic, the most famous is that in its form of oxide As 2O 3 : arsenic trioxide. The latter is responsible for many famous poisonings in history, it is also part of the components of death to rats.
Arsenic trioxide is in the form of a white, odorless and tasteless powder. In the Middle Ages it was the favorite poison of the murderers, it was also very used to the renaissance in particular among the Borgia (Italian family very influential in the fifteenth century).
Arsenic trioxide: As 2 O 3
wikipedia
This poison was very interesting in the Middle Ages because its symptoms are similar to those of Cholera that raged at the time. It is even given the nickname of "powder of succession" because of its frequent use to accelerate the possible successions.
Symptoms
Inorganic arsenic (in the form of oxide, salts or ions) is essentially absorbed orally. It is then transferred into the bloodstream and distributed rapidly to all organs (especially the liver and kidneys).
Symptoms of acute ingestion include:
- vomiting
- Paralysis
- Abdominal pain
- Bloody diarrhea
- Sensation of dry throat
- convulsions
- Delusion and death
These symptoms occur rapidly, within hours of ingestion.
Arsenic trioxide label
www.ineris.fr
For acute intoxications (very high dose for a short time), death may occur rapidly, a few hours after ingestion. The lethal dose in humans is estimated to be between 1 and 3 mg / kg / d.Which means it takes to kill a man of about 80 kg, administering about 240 mg of arsenic in one day.
Action plan
It is important to note that inorganic arsenic is predominantly present in oxidation states III and V. For Arsenic trioxide, for example, the arsenic atom is in the oxidation state III. Arseniates (As III) and arsenites (As V) do not interact in the same way on the human corpus, but they are both toxic. However, arsenate is 60 times more toxic than arsenite.
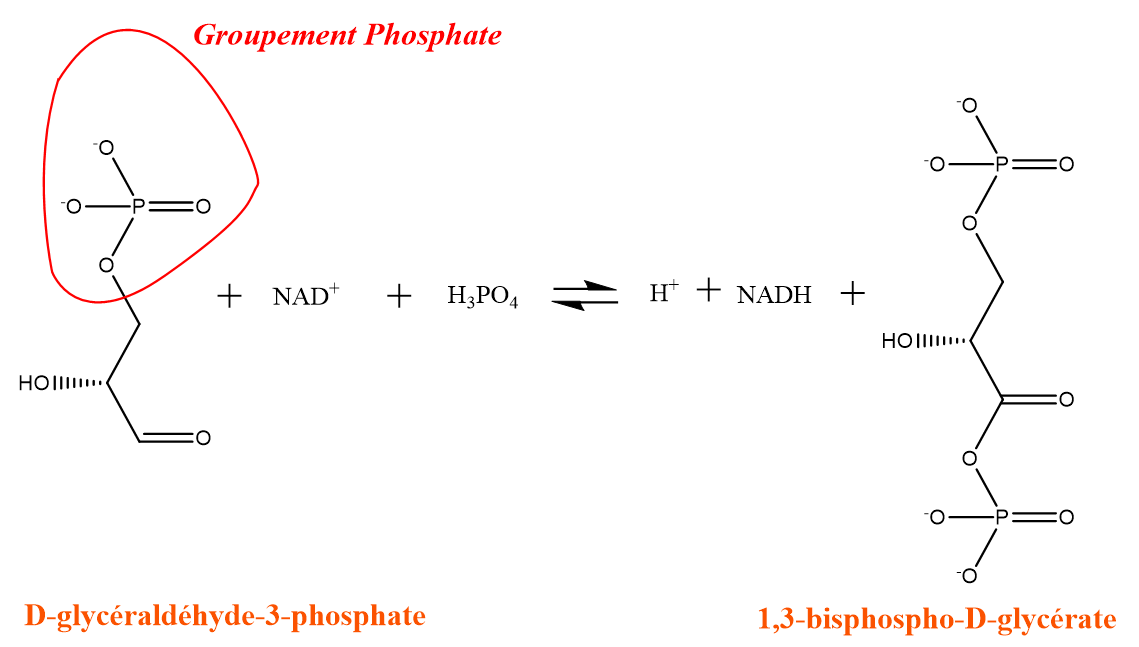
As (V) is toxic because of its reduction in As (III) in the human body. Arsenic (III) is very chemically close to Phosphorus. These two elements are also located in the same column of the periodic table (column 15). They are both capable of forming five bonds, so that arsenic (III) can replace phosphates (R-PO 4 2- group ) in glycolysis reactions (assimilation of glucose to produce energy) and Synthesis of ATP ("energy currency" of the body). These last two reactions were then inhibited by arsenic.
Phase 3 of glycolysis: recovery of energy by phosphorylation
The As (III), for its part, will inhibit the pyruvate dehydrogenase complex which catalyzes the transformation of pyruvic acid into acetyl co-enzyme A. This latter is necessary for the formation of ATP. The direct consequence of this inhibition is a decrease in the production of ATP.
Formation of acetyl co-enzyme A catalyzed by the pyruvate dehydrogenase complex
Arsenic therefore causes a decrease in ATP in the body and, knowing the major role of ATP in the various metabolisms of the human body, this explains the harmful consequences of the ingestion of a " arsenic.
Bibliography
-
Wikipedia
-
http://www.inrs.fr/publications/bdd/fichetox/fiche.html?refINRS=FICHETOX_89§ion=pathologieToxicologie#tab_toxiHomme
Other articles
Inscrivez-vous au blog
Soyez prévenu par email des prochaines mises à jour
Rejoignez les 13 autres membres