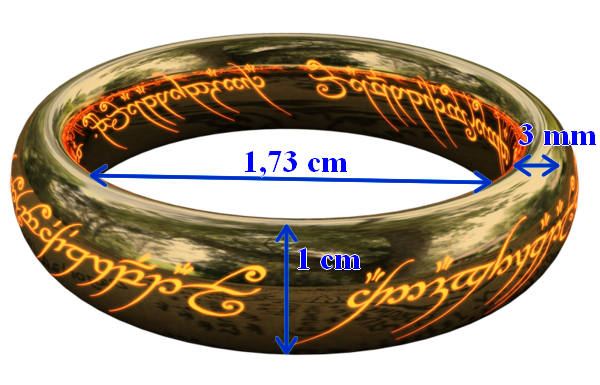
The one ring !
"A ring to rule them all, a ring to find them, a ring to bring them all and in darkness to bind them . "
The unique ring of the lord of the rings possesses a number of amazing properties: conferring invisibility, it can not be scratched or damaged, and it can only be destroyed in the flames of the mountain of destiny. But a question then chokes the mind of the chemist: of what metal is the ring made? Does this explain some of its properties? Let's study the unique ring, chemistry in support!
History of the ring
Properties of the single ring
- It is impossible to destroy except in the fire of the mountain of destiny
- It can not be scratched, damaged
- It prolongs the life of its wearer, but the latter becomes more and more weary
- He is able to stir up his desire
- It adapts to the finger of the wearer
Small summary of the trip of the ring
- It is forged by Sauron in the mountain of destiny
- Isildur recovers the ring by cutting the finger of Sauron
- The ring is lost in the river Anduin for two thousand years
- A hobbit finds the ring and becomes a solitary creature, and schizophrenic: Gollum
- The ring is preserved by Gollum for five hundred years in a cave
- Bilbo Sacquet recovers the ring and then bequeaths it to Frodo Sacquet
- The latter has the mission of bringing the ring to the mountain of destiny to destroy it
In the Lord of the Rings universe it seems that the ring is made of gold: is this coherent?
The Colour
The color of the ring perfectly matches the color of the gold: yellow.
The density
Gold is a metal and therefore has a fairly high density: d = 19.32 . It is recalled that the density is the ratio between the density of the compound (here the gold) and the density of a reference compound (here water). To give an idea, a density of 19.3 means that a solid gold tennis ball would weigh 2.6 kg: much less practical for smash!
Considering the following dimensions for the ring, a gold volume of 1.91 cm 3 (1.91 mL) is obtained and therefore a mass of 37 g .
wikipedia
So despite the heavy burden that this ring represents and all the weight that seems to carry Frodo, this ring does not really weigh so heavy.
Malleability and hardness
Gold is a very malleable and highly ductile material, so it can be deformed by hammering and stretching even when cold. It should be noted that one of the characteristics of the ring is that it is able to adapt to the finger of its wearer: Sauron and Frodo do not indeed have exactly the same finger width. One can then try to explain this phenomenon by this capacity that has gold to be able to easily deform.
Gold is not a very hard metal. The hardness is the resistance of one material to be scratched or not by another. Diamond is an extremely hard material, it can only be scratched by another diamond. A scale based on comparisons between metals exists: the Mohs scale, ranging from 1 (very hard as talc) to 10 (the diamond).
The gold has a hardness of 2.3 , so it can be scratched by a piece of copper, a knife, a sword ....
This does not correspond at all to the characteristics of the single ring which can not be scratched or damaged. We have, for example, this very well-known scene where Gimli gives a great blow of an ax in the ring.
Gimli tries to destroy the ring at 12 seconds.
Toxicity
Gold is not toxic by simple contact with the human body. This does not explain the health of Frodo, which is deteriorating in the course of history.
Oxidation
The ring in the film is incredibly resistant. He spent two thousand years at the bottom of a river, then five hundred years in a cave. The latter is therefore constantly exposed to moisture, yet unlike many metals it does not corrode, oxidize and remains intact.
Gollum and his ring in the cave
webdesign.mistercaldwell.com
This property can be explained by a very interesting characteristic of gold: it is stainless.Indeed, when it is specified in chemistry that a compound is stainless, it means that the compound can not be oxidized by water or by the oxygen in the air.
Oxidation of metals in chemistry corresponds to the transformation of metal M into a species that has lost one or more electrons. This new species can be in the ionic form M n + (as Fe giving Fe 3+ ) or in the oxide form: M m O n (as Fe giving the rust Fe 2 O 3 ). Oxidation of metals is often considered deterioration and can be caused by water or oxygen from the air.
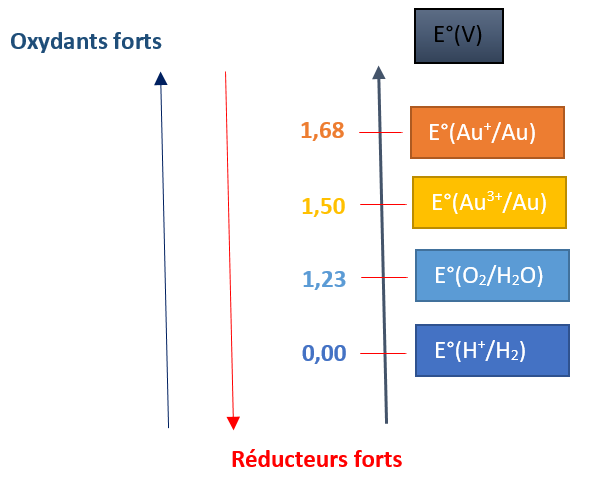
To know whether a species will oxidize a metal or not, we must compare the electrical potential potentials of the species involved: denoted E ° and expressed in volts.
The redox couples are then classified according to their standard potential E ° (ox / red), they correspond to the potential difference between a half-cell constituted by the torque studied and another half-cell constituted by the reference torque H + / H 2 .
To oxidize the gold a species is needed which is more oxidizing, therefore that has a potential higher than 1.50 V (to form Au 3+ ). However, water (H + / H2 couple) and dioxygen (O2 / H2O) have a lower potential than gold pairs and therefore can not oxidize it.
Most metals are oxidized by water and dioxygen because they typically have much lower standard potentials. With the exception of gold, silver and some other (noble metals) which are therefore stainless.
This is why despite all the years spent in humidity, the water and mud ring is still intact and has not corroded.
Bibliography
- http://www.societechimiquedefrance.fr/or.html
- http://culturesciences.chimie.ens.fr/content/lor-cyanuration-dissolution-de-lor-par-leau-892
- http://www.periodni.com/fr/au.html
- Wikipedia
Other articles
Inscrivez-vous au blog
Soyez prévenu par email des prochaines mises à jour
Rejoignez les 13 autres membres