Chloroformed Tintin
The secret of the unicorn, Hergé The black island, Hergé
Hello everyone :)
About the day: an intrepid journalist, chloroform and chemistry!
In the adventures of Tintin, the hero of our youth never ceases to be mistreated by his many adversaries. He will be subjected to numerous blows of clubs, batons and other bars of iron.But what prompted me very quickly is the very present use of chloroform by those pesky "waffle molds" that are the bad guys.
A small description of chloroform
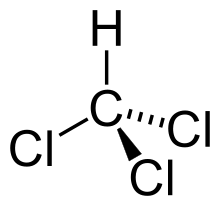
Chloroform: CHCl 3
www.wikipedia.fr
Chloroform, of the formula CHCl 3 (or trichloromethane) is a colorless and highly volatile liquid. It has been widely used as a solvent in laboratories and as anesthetic in operating theaters. Between 1865 and 1920 it was used in 80 to 95% of the anesthesia carried out in Great Britain and in the Germanic countries. For the record: it was used in particular for the birth of the last two children of Queen Victoria. [2]
Chloroform in a test tube Bottle of chloroform
Www.wikipedia.co.uk www.pinterest.com
Its use has, however, been greatly reduced since the 1920s, caused by the arrival of new anesthetics (nitrous oxide, hexobarbital) and especially by the discovery of the significant toxicity of this compound.
Toxicity of chloroform
Symptoms following inhalation are:
- Increased respiratory rate then decreased during deep anesthesia
- Gastrointestinal effects: may cause nausea and vomiting
- Toxic to the heart, liver and neurons
- Carcinogenic compound
- AT High concentrations can lead to fatigue, dizziness and headache
To produce narcosis (artificial sleep by administration of a substance) an average concentration of 15 000 ppm is required. The ppm is a quantity used in science to express concentrations for very small amounts of compound. Ppm means "Part Per Million", so 1 ppm represents 1 compound (molecule, atom, ion, ...) present among 1 million other compounds. In our case we are interested in gases therefore 1 ppm = 1 millionth of liter in 1 liter of inspired air, so 1 ppm = 1 μL per liter of air.
Thus, to cause a coma, this corresponds to about 15 L of gaseous chloroform per cubic meter of air , or even 76 g of gaseous chloroform per cubic meter of air.
For those interested, a small exercise is proposed at the end to determine the volume of liquid chloroform that must be evaporated to be able to sleep Tintin.
Very high doses of inhaled chloroform can also lead to death. (Greater than 40,000 ppm).
In the body the chloroform will mainly affect the tissues with a large amount of lipids. Indeed chloroform is lipophilic, it means that it has a good affinity with fatty compounds such as lipids and fats ... So this is why it affects especially the brain, the liver and the fats.
Chloroform in Tintin
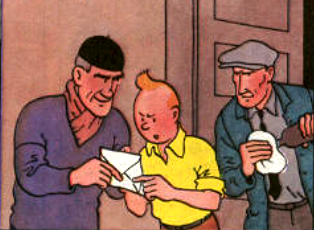
Here is an excerpt from the black island, in this last Tintin trying to escape his opponent by locking himself in a room (maybe not the best idea that he had this shot there) containing medical equipment. The repeated shots of its assailants ended by breaking a bottle of chloroform placed, as the standards of safety in the laboratory, in the corner at the top of a shelf. After a few minutes Tintin finally fainted in a deep coma ... Let's study this chemistry extract in support.
What is the problem here?
Since its widespread use as anesthetic, chloroform has been very much sought after in the cinema by thieves, murderers and kidnappers in order to neutralize their victim. The classic technique that all of us have in mind is to force the victim to breathe a cloth soaked with chloroform and thus cause an instantaneous fainting.
Articles from medical journals have been published to explain that this belief is false [1].Indeed, although it is true that chloroform has served as an anesthetic and can actually cause fainting, its effects are by no means almost instantaneous. Usually about 5 minutes are needed to produce an anesthetic effect, then the application must be continued for the effects to last. Moreover, if one does not wish to kill one's victim, one must also be careful to keep one's chin firmly, so that it does not suffocate with its tongue.
Thus it is immediately much more difficult to imagine a criminal apply for 5 minutes a rag on the nose of a victim quite able to resist and struggle during this period of time.
In the preceding excerpt, the reasoning is the same, Tintin vanishes too quickly. Despite the fact that it is not a simple chloroform rag but a whole bottle that breaks, Tintin still had time to find a way to escape before fainting.
Article reference :
- Ineris chloroform sheet: chloroform.pdf
- Wikipedia Chloroform (English page) [2]
- www. Chromepharmacy
- The criminal use of chloroform, JP Payne
- Chloroform among thieves, lancet 1805 [1]
Other articles
Inscrivez-vous au blog
Soyez prévenu par email des prochaines mises à jour
Rejoignez les 13 autres membres