Gandalf the White
Gandalf the White, the Return of the King
- "Hahahaha, you have no power here Gandalf the gray" (Theoden in old schnock mode)
- (small sacred music "haaaaaaaa!")
- Hahaa! And bim in your face Sarouman, my clothes are of a brilliant whiteness (Gandalf)
- "Oh no, it's not possible! How does he get such white clothes ?!" (Saruman)
- And boom ! Stick hit !
That's the whole question, how does Gandalf go from gray to bright white? In the book it is stated that "it is sent back to earth because its task is not finished", but before this explanation a little idle the chemist sneers gently and a word comes to him in head: flavors !
White
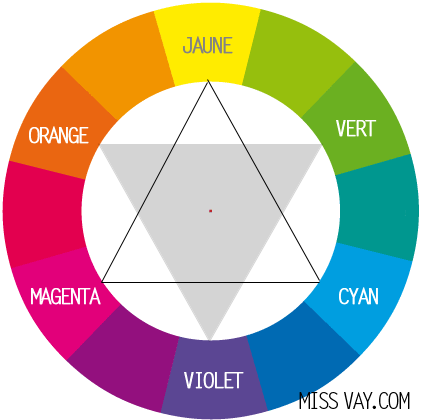
First of all let us specify what "white" is : a mixture of all colors. When the light arrives on an object, so that the latter appears to us white, it must therefore return all the wavelengths of the visible spectrum (hence all colors) to our eyes.
If an object absorbs a color of the visible spectrum, the complementary color appears. And if it absorbs all the colors it is black. We can therefore determine the colors that an object absorbs thanks to the chromatic circle.
The absorbed color is the opposite of the color that is observed :
"But tell me, Jamy, what connection with our dear friend Gandalf ?"
- Well it's very simple ...
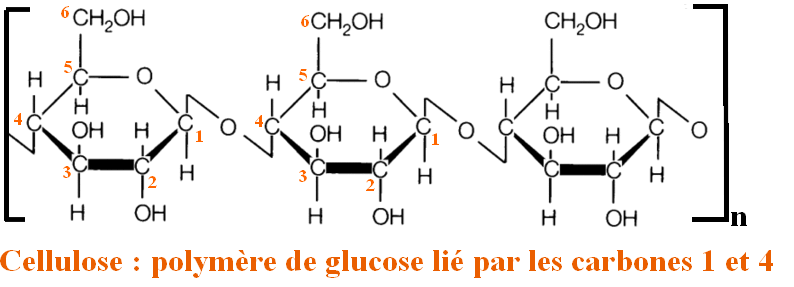
The concern our friend Gandalf should have is that his clothes are made of cotton. Cotton is composed essentially of cellulose : a polymer (repeat of the same chemical motif) of D-glucose.
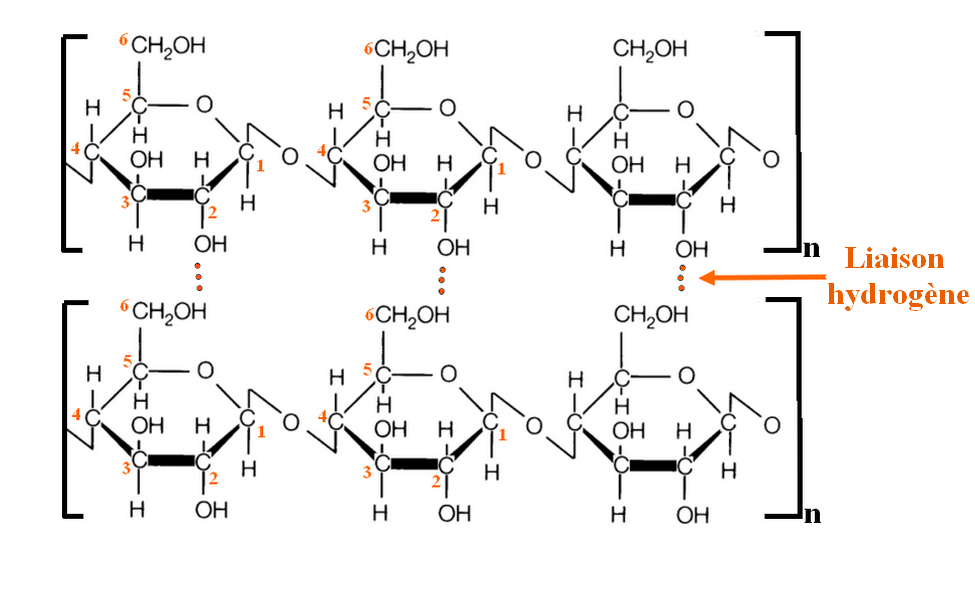
The fibers of cotton come from these long chains, linked together by hydrogen bonds (an electrostatic bond between a hydrogen and an oxygen here, a little less strong than a real covalent bond).
The main concern of cotton is that it absorbs in the blue, so normally all our clothes made of cotton should appear to us yellowish (complementary color of the purple-blue).
To overcome this problem, our friend Gandalf, really ahead of his time well taken care to add optical brighteners to his beautiful white cape.
The brighteners
The optical brighteners are compounds which are added to the garments (about 1% by weight), so that the latter appear white and not yellowish, or even "whiter than white".
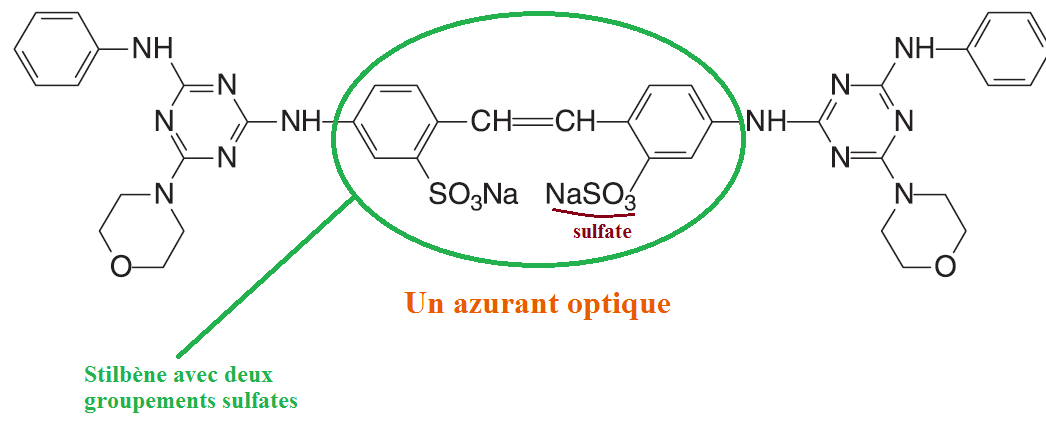
The brighteners are often stilbene derivatives and possess sulphate groups. These groups make the polar molecule and therefore ensure it a good solubility in water (which is important because the brighteners are widely used in detergents).
Moreover, these sulfate groups will allow the brightener to bind to the cellulose via hydrogen bonds (again and again). The bonds are made between the oxygens of the brightener (sulfate group) and the hydrogens of the alcohols of the cellulose.
The brightener has this ability to absorb light in the ultraviolet (it is therefore colorless). This absorption gives it an important energy: the molecule is then in an excited state and will thus be able to release its surplus energy in the form of heat or by emitting light: it is fluorescence!
The brighteners are therefore fluorescent molecules: they can therefore emit light and in particular blue light (around 400-500 nm), thus completing the missing color in the spectrum that the cellulose has absorbed.
But this is not all, for in addition to returning all the colors, the brightener emits light : hence this bright white, which is often called "whiter than white".
Laundry with optical brighteners
As a bonus, this is the famous sketch by Coluche, where he laughs at washing powders that wash "whiter than white" !
Bibliography
- Wikipedia for optical brighteners
- Wikipedia cellulose
- Articles of the chemical news on the laundry
Other articles
Inscrivez-vous au blog
Soyez prévenu par email des prochaines mises à jour
Rejoignez les 13 autres membres